Welcome to the website of the Creative Design Studio

Rechargeable aqueous electrochemical energy storage (EES) devices, especially ones using earth-abundant and non-toxic electrode materials and Na-ions as charge carriers, have shown great promise for various applications for their low cost and environmental friendliness. Our research group has been focusing on the synthesis, characterization and test various nanostructured electrode materials (Figure above), with the emphasis on gaining fundamental understanding of charge transport process of electrode materials using ex situ and in situ photon (e.g., synchrotron), electron (e.g., electronic microscopy), neutron, and electrochemical tools. Our research objective is to design electrode materials able to deliver high energy and power densities for use in aqueous EES devices that can rival the performance of their non-aqueous Li-ion counterparts.
Thrust 1: Fundamental Studies of Electrode/Electrolyte Interface for Aqueous Electrochemical Energy Storage
Thrust 2: Ethanol Chemistry: Utilizing Ethanol as Feedstock in Electrochemical and Hetergeneous Catalytic Processes for Energy and Chemicals Generations
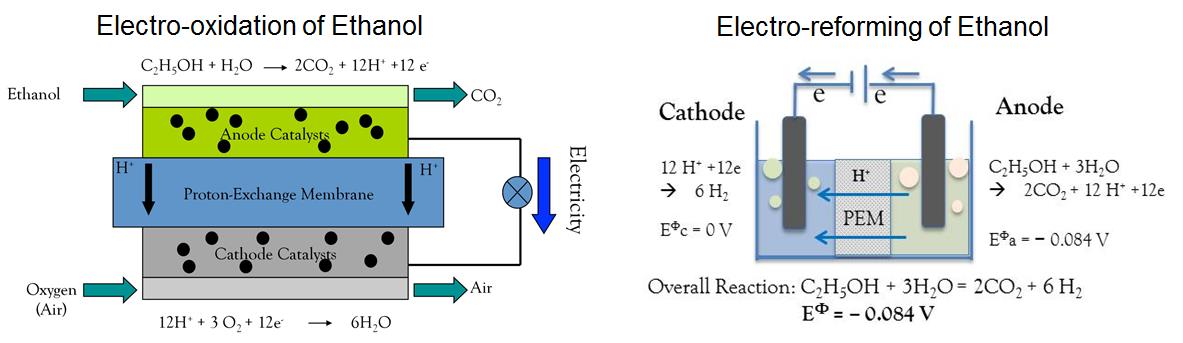
Ethanol has been considered a promising fuel in fuel cell technologies for its high energy density, low toxicity and availability from biomass. However, ethanol oxidation reaction (EOR, CH3CH2OH + 3H2O à 2CO2 + 12H+ + 12 e–) at the anode is plagued by slow kinetics and inefficient oxidation of ethanol into CO2 via C–C bond–breaking (Figure above left). Our research objective is to understand the reaction pathways of EOR on various surfaces, and then design effective EOR catalysts that have fast reaction kinetics and good capability to split C–C bond of ethanol molecule to achieve 12–electron charge transfer.
Moreover, our group is interested in an electrochemical reforming process that converts ethanol to hydrogen (Figure above right). During the ethanol electrochemical reforming (EER), ethanol reacts with water and forms carbon dioxide, protons and electrons at anode via EOR at anode, and hydrogen is produced through hydrogen evolution reaction (HER) in acid environment at cathode. It is clear that overall cell voltage of EER at equilibrium is only –0.084 V. Theoretically, nearly 100% electrical energy can be provided for hydrogen generation by electrochemical reforming process. This is in a sharp contrast to water electrolysis, in which the oxygen evolution reaction (OER) at anode consumes most of electricity provided for the cell (1.23 V). Moreover, OER at anode intrinsically has large overpotential, requiring a large amount of energy to overcome the kinetics barrier. Therefore, water electrolysis requires much higher electrical energy compared with EER for the same hydrogen yield.
Moreover, our group is interested in an electrochemical reforming process that converts ethanol to hydrogen (Figure above right). During the ethanol electrochemical reforming (EER), ethanol reacts with water and forms carbon dioxide, protons and electrons at anode via EOR at anode, and hydrogen is produced through hydrogen evolution reaction (HER) in acid environment at cathode. It is clear that overall cell voltage of EER at equilibrium is only –0.084 V. Theoretically, nearly 100% electrical energy can be provided for hydrogen generation by electrochemical reforming process. This is in a sharp contrast to water electrolysis, in which the oxygen evolution reaction (OER) at anode consumes most of electricity provided for the cell (1.23 V). Moreover, OER at anode intrinsically has large overpotential, requiring a large amount of energy to overcome the kinetics barrier. Therefore, water electrolysis requires much higher electrical energy compared with EER for the same hydrogen yield.
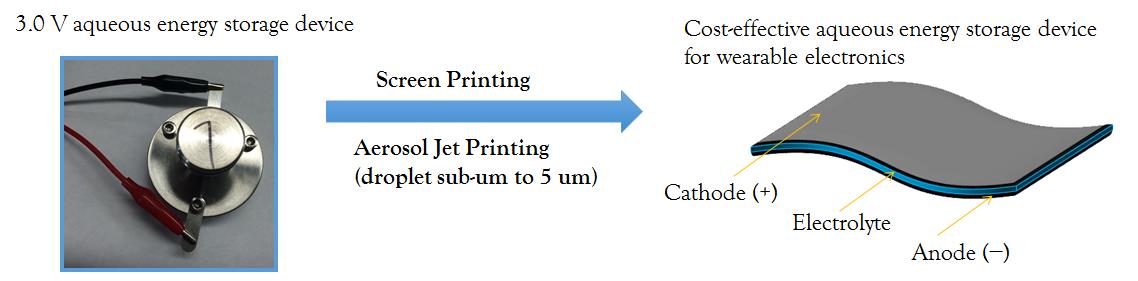
Thrust 3: Energy Storage + Printing = Flexibale & Wearble Electronics
Wearable electronics is a major growth area for thin film and flexible batteries. Most of the conventional rechargeable batteries struggle to meet the requirement for wearable electronics in terms of flexibility, thinness, light weight and safety. Our group plans to combine our expertise in aqueous energy storage and printed electronics to develop thin film and flexible aqueous EES devices for applications in wearable electronics. In addition to screen printing, additive manufacturing (or 3D printing) method has been considered. Our goal is to design a paradigm of thin film EES devices fabricated by printing techniques with our newly developed electrode materials, to provide high volumetric energy density and power density performance for wearable electronics.